"Junk DNA" guides Epigenetic Transgenerational Inheritance
Article; Small RNAs in the Transgenerational Inheritance of Epigenetic Information, Duempelmann et al. (1/21)
Abstract
Epigenetic information, which refers to heritable changes in gene expression that do not alter the DNA sequence as with neo-Darwinism, is increasingly recognized as a key player in development. Small RNAs, a class of non-coding RNAs that are involved in RNA interference (RNAi), have emerged as important mediators of epigenetic inheritance.
Sadly due to NeoDarwinian theory the Junk DNA where nc RNA came from was ignored for 30 years.
In this review authors highlight mechanistic studies in model organisms that advance our understanding of how small RNAs trigger long-lasting epigenetic changes in gene expression and discuss observations that lend support for the idea that small RNAs might participate in mechanisms that trigger epigenetic gene expression changes in response to environmental cues and the effects these could have on population adaptation.
Introduction
Epigenetic information, which refers to heritable changes in gene expression that do not alter the DNA sequence as opposed to NeoDarwinism, is increasingly recognized as a key player in development. Epigenetic modifications, such as DNA methylation and histone modifications, can alter chromatin structure and accessibility, thereby influencing gene expression patterns. While epigenetic modifications are typically considered to be reversible, there is growing evidence that epigenetic information can be stably inherited across generations, leading to transgenerational epigenetic inheritance (TEI).
Small RNAs, a class of non-coding RNAs that are involved in RNA interference (RNAi), have emerged as important mediators of TEI. RNAi is a mechanism by which small RNAs target and silence complementary messenger RNAs (mRNAs), leading to the suppression of gene expression. Small RNAs can also direct the deposition of epigenetic marks, such as DNA methylation and histone modifications, on target genes. These epigenetic marks can be stably inherited across generations, leading to long-lasting changes in gene expression.
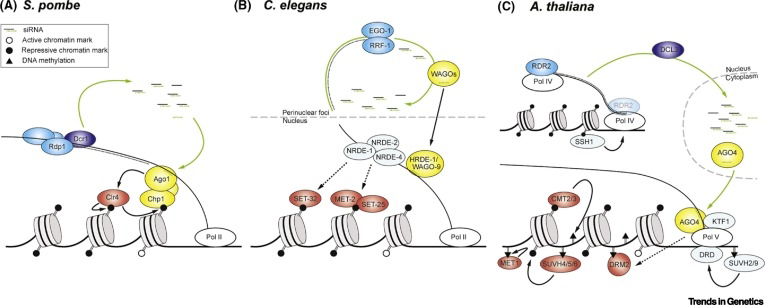
Mechanistic Studies of Small RNA-Mediated TEI
Mechanistic studies in model organisms have provided valuable insights into how small RNAs mediate TEI. In Drosophila melanogaster, small RNAs can be inherited across generations through the female germline. These small RNAs can then target and silence genes in the offspring, leading to changes in gene expression patterns. For example, small RNAs derived from the maternal germline can silence the paternal copy of the X chromosome in female offspring, a process known as X chromosome inactivation.
In Caenorhabditis elegans, small RNAs can be inherited across generations through both the male and female germline. These small RNAs can then target and silence genes in the offspring, leading to changes in gene expression patterns. For example, small RNAs derived from the paternal germline can silence genes involved in metabolism in offspring, leading to changes in body size.
In mammals, the role of small RNAs in TEI is less well understood. However, there is evidence that small RNAs can be inherited across generations in mammals, and that these small RNAs can target and silence genes in the offspring. For example, small RNAs derived from the maternal germline can silence genes involved in behavior in offspring, leading to changes in behavior patterns.
Small RNAs and Environmental Adaptation
Observations from model organisms suggest that small RNAs might participate in mechanisms that trigger epigenetic gene expression changes in response to environmental cues. These epigenetic changes could then be inherited across generations, leading to adaptations that help populations cope with changing environments. For example, in Drosophila, exposure to stress can lead to the production of small RNAs that target and silence genes involved in stress resistance. These epigenetic changes can then be inherited across generations, leading to increased stress resistance in offspring.
Conclusion
Small RNAs have emerged as important mediators of TEI. Mechanistic studies in model organisms have provided valuable insights into how small RNAs trigger long-lasting epigenetic changes in gene expression. Observations from model organisms also suggest that small RNAs might participate in mechanisms that trigger epigenetic gene expression changes in response to environmental cues. These epigenetic changes could then be inherited across generations, leading to adaptations that help populations cope with changing environments. Further research is needed to fully understand the role of small RNAs in TEI and their potential impact on adaptation.
Challenging Neo-Darwinian Orthodoxy
The transgenerational inheritance of epigenetic information mediated by small RNAs challenges the Neo-Darwinian view of inheritance. Under Neo-Darwinian principles, inheritance is solely determined by the transmission of DNA mutations, which are then subjected to natural selection. However, the ability of small RNAs to transmit acquired epigenetic marks suggests that environmental factors can also play a significant role in shaping the evolution of organisms.
This raises questions about the relative contributions of genetic and epigenetic factors in evolutionary processes. It also suggests that environmental changes can have far-reaching consequences, influencing the traits of future generations even in the absence of direct DNA sequence alterations.
The discovery of small RNA-mediated transgenerational inheritance has revolutionized our understanding of inheritance and evolution. It has challenged Neo-Darwinian orthodoxy by demonstrating that environmental factors can influence gene expression and phenotypic traits without altering the underlying DNA sequence. Further research into this complex phenomenon is crucial for understanding the mechanisms underlying transgenerational inheritance and its implications for human health and disease.
For now it's clear neo-Darwinism needs revision if not replacement.



Re: "Sadly due to NeoDarwinian theory the Junk DNA where nc RNA came from was ignored for 30 years." It's been 27 years since my group linked Darwin's "conditions of life" to light-activated miRNA-mediated pH-dependent protection from the virus-driven degradation of mRNA via God's Creation of sunlight and humidity at the origin of life ~6000 years ago. See: "From Fertilization to Adult Sexual Behavior" http://www.hawaii.edu/PCSS/biblio/articles/1961to1999/1996-from-fertilization.html (1996)
ReplyDelete