Calling the Question: What is Mammalian Transgenerational Epigenetic Inheritance?
The intricate dance of life involves a complex interplay between genes and environment. While genes provide the blueprint for an organism, the environment can influence how these genes are expressed. This modulation of gene expression, independent of changes in the DNA sequence itself, is epigenetics. But the story doesn't end there. Recent research suggests that these environmentally induced epigenetic changes can be passed down through generations, a phenomenon known as transgenerational epigenetic inheritance
In mammals, this field of study is still in its infancy, yet it holds immense potential for our understanding of how past experiences can shape future generations.
Before delving into the specifics of mammalian transgenerational epigenetic inheritance, let's establish a foundational understanding of epigenetics. Epigenetic modifications act as chemical tags attached to DNA or the proteins packaging it (histones). These tags don't alter the DNA sequence but can influence how tightly genes are wound, essentially regulating their accessibility. Tightly wound DNA is less accessible for transcription (the process of copying DNA into RNA), leading to decreased gene expression. Conversely, looser packaging allows for easier access and increased gene expression.
Traditionally, these epigenetic modifications were thought to be erased during germline development (sperm and egg cell formation), ensuring a clean slate for each generation. However, recent evidence suggests that these modifications might not be entirely wiped clean. This persistence of epigenetic information across generations is what we refer to as transgenerational epigenetic inheritance.
In mammals, research on transgenerational epigenetic inheritance is ongoing and complex. Examining epigenetic marks in sperm and egg cells is challenging, as these cells are terminally differentiated (cannot divide further). Additionally, disentangling the influence of the maternal environment during pregnancy from true transgenerational inheritance adds another layer of complexity.
Despite these challenges, studies have demonstrated transgenerational epigenetic inheritance in mammals. For instance, a study in mice demonstrated that exposure to a high-fat diet in one generation could lead to changes in the offspring's metabolism, even if the offspring itself was fed a normal diet. These changes were associated with epigenetic modifications in the sperm of the exposed fathers. Similarly, studies in rats suggest that exposure to stress in one generation can influence stress responses and behaviors in subsequent generations.
These findings require further investigation to establish transgenerational epigenetic inheritance in mammals. Future research needs to address challenges like isolating true transgenerational effects from maternal influences and identifying the specific epigenetic mechanisms involved. Additionally, studies need to explore the long-term health consequences of these inherited epigenetic modifications.
Understanding transgenerational epigenetic inheritance has significant implications. It could explain how environmental exposures, such as diet, toxins, or stress, can have lasting effects on future generations, even in the absence of direct exposure. This knowledge could be crucial for developing preventative measures to mitigate the health consequences associated with these exposures.
For example, if certain environmental exposures are found to induce detrimental epigenetic changes that are then passed down, interventions could be targeted towards preventing these changes in the first place. Additionally, understanding how these epigenetic modifications influence health could lead to the development of therapies aimed at reversing or mitigating their effects.
The field of transgenerational epigenetic inheritance in mammals is still evolving. While the evidence is suggestive, more robust research is needed to solidify the concept and understand its mechanisms. However, the potential implications for our understanding of health and disease across generations are undeniable. As we continue to unravel this intricate dance between genes, environment, and epigenetics, we might be unlocking new avenues for promoting health and well-being for generations to come.
The Enigma of Epigenetic Inheritance: A Challenge to Neo-Darwinism?
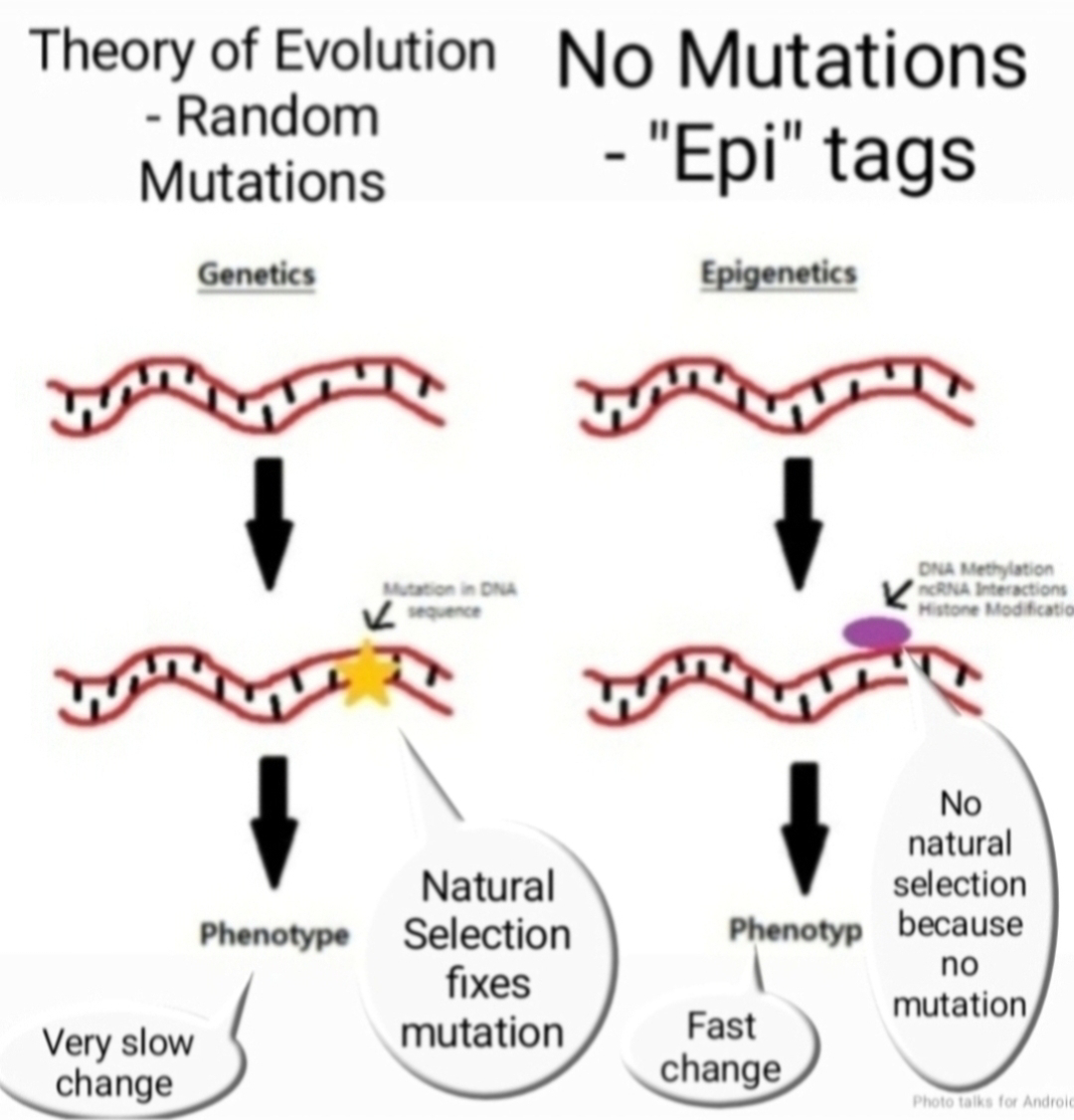
The concept of inheritance has long held the DNA sequence as the sole player. However, a new contender has emerged: transgenerational epigenetic inheritance (TEI) in mammals. TEI proposes that environmental factors can influence gene expression in offspring, bypassing changes in the DNA code itself. This phenomenon throws a curveball at Neo-Darwinian theory, which emphasizes genetic variation and natural selection as the driving forces of evolution.
The controversy surrounding TEI in mammals stems from two key challenges. Firstly, mammals undergo two "epigenetic reprogramming" events during development – one in germ cells and another in the embryo. These events essentially wipe the slate clean of epigenetic marks, making it difficult for modifications to persist across generations. Secondly, distinguishing TEI from intergenerational effects (where environmental factors directly affect the developing offspring in the womb) is crucial, as it's the transmission across multiple generations that truly challenges Neo-Darwinism.
Despite these hurdles, evidence for TEI in mammals is accumulating. Studies on coat color in mice and the effects of stress on offspring behavior hint at the possibility. If confirmed, TEI suggests that environmental influences can shape the health and characteristics of future generations, even without altering the DNA sequence itself. This expands the definition of inheritance beyond the classical neo darwinian DNA-based model.
Neo-Darwinism focuses on random mutations in DNA and their subsequent selection by the environment. TEI, on the other hand, implies a more nuanced interplay between environment, gene expression, and inheritance. It suggests that environmental cues can leave a lasting mark on the epigenome, potentially influencing the evolution of populations over time.
TEI challenges Neo-Darwinism and compels a broader view of inheritance. It raises questions about the extent of environmental influence on evolution and the potential for adaptation beyond pure genetic changes. Further research on TEI in mammals holds the key to unlocking a deeper understanding of how life evolves and inherits traits across generations.






Comments
Post a Comment